Synthetic biology for sialyllactose production


GALAB focuses on the development of economical and safe production processes for human milk oligosaccharides (HMO), which play an important role in the early development of infants. In this way, GALAB aims to contribute to the optimal nutrition of infants who cannot be breastfed.
Sialylated HMOs such as sialyllactose (SL) are important components of breast milk and ensure that toxins are neutralised and the adhesion of bacteria and viruses to the epithelial surface is prevented when infants are breastfed. Additionally, HMOs are needed for research in diseases (infectious, autoimmune and cancer diseases) and for use in pharmaceuticals and biologically functional materials 1-5.
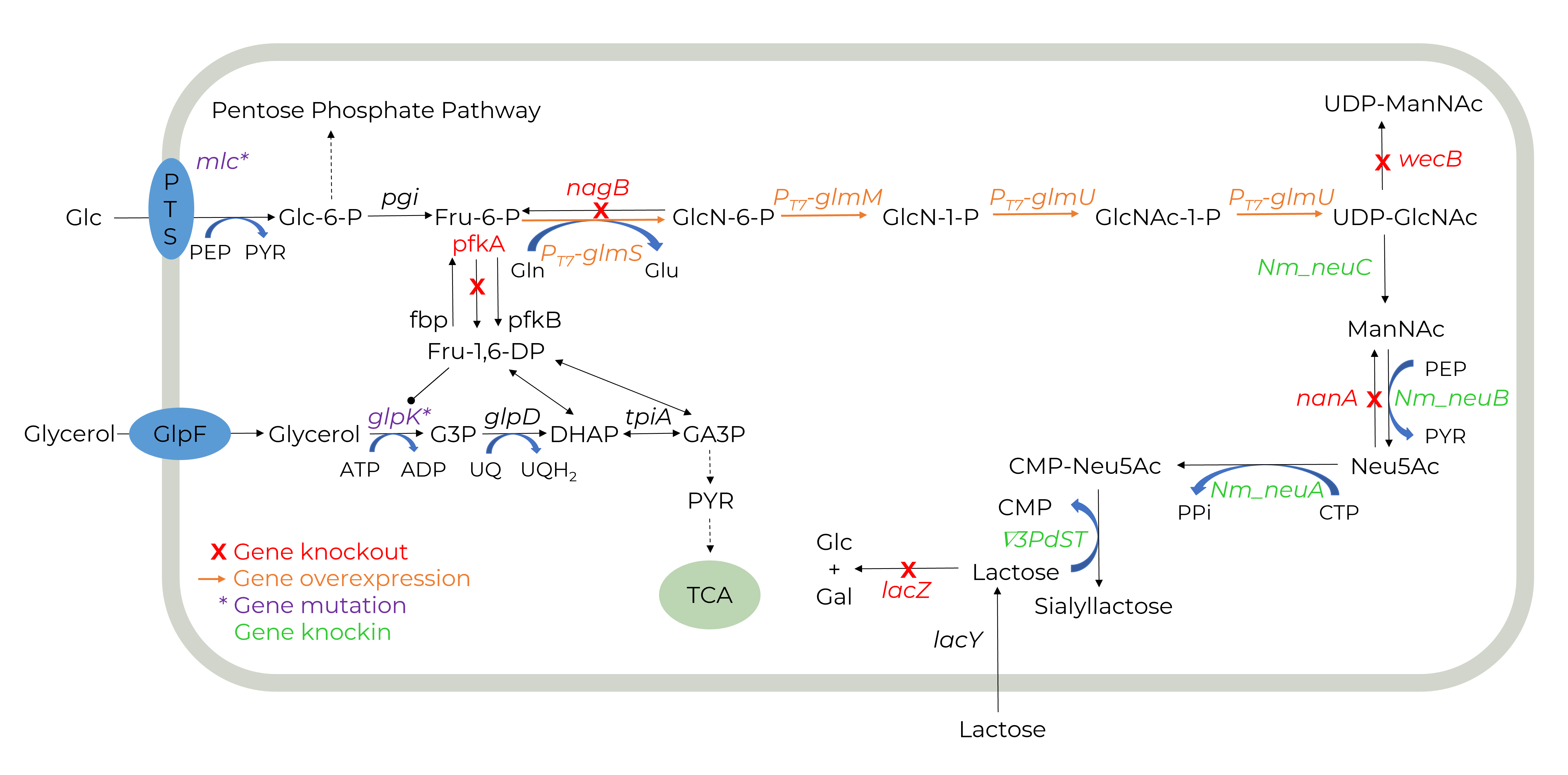
GALAB develop a biotechnological production process in which genetically modified bacteria are used in fermentation processes to express the necessary enzymes for SL production.
The use of whole cell biocatalysts in the production of SL has several advantages. Whole cells are a cost-effective form of biocatalysts, and the cell wall has a natural protective function for enzymes. In addition, time-consuming steps can be avoided, and the regeneration of cofactors is possible, leading to a reduction in costs.
The main challenges in the strain development of a whole-cell biocatalyst for HMO production are the need for multiple enzymes, cofactor regeneration, substrate uptake and product release, and the prevention of degradation of intermediates.
We develop the SL-producing strain using the modern genome editing technique CRISPR-Cas9. Another aspect considered in strain construction is the transport pathway of substrates and product into/out of the cell, overcoming previous limitations.
Sources
1 Z. Li, et al., “Multi-Enzymatic Cascade One-Pot Biosynthesis of 3’-Sialyllactose Using Engineered Escherichia coli,” Molecules, vol. 25, no. 3567, 2020.
2 K. Schmölzer et al., “Complete switch from α-2,3- to α-2,6-regioselectivity in Pasteurella dagmatis β-d-galactoside sialyltransferase by active-site redesign,” Chem. Commun., vol. 51, no. 15, pp. 3083–3086, 2015, doi: 10.1039/C4CC09772F.
3 L. Guo, et al., “Enzymatic Synthesis of 6’-Sialyllactose, a Dominant Sialylated Human Milk Oligosaccharide, by a Novel exo-α-Sialidase from Bacteroides fragilis NCTC9343,” Appl. Environ. Microbiol., vol. 84, no. 13, pp. e00071-18, 2018, doi: 10.1128/AEM.00071-18.
4 L. E. Horsfall, et al., “Identification and characterization of important residues in the catalytic mechanism of CMP-Neu5Ac synthetase from Neisseria meningitidis,” FEBS J., vol. 277, no. 13, pp. 2779–2790, 2010.
5 S. Schelch, et al., “Engineering analysis of multienzyme cascade reactions for 3’-sialyllactose synthesis,” Biotechnol. Bioeng., vol. 118, pp. 4290–4304, 2021, doi: 10.1002/bit.27898.